
This site is intended for US healthcare professionals only.
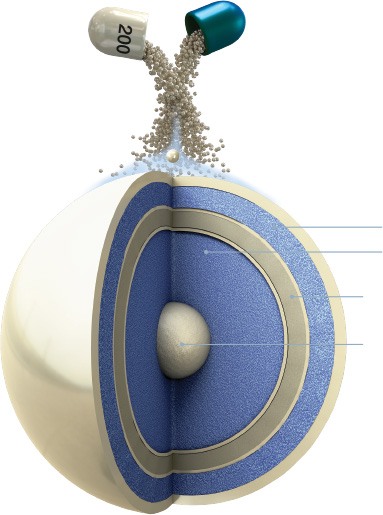
First layer of lacosamide releases immediately upon capsule breakdown
XR coating controls the release rate of remaining lacosamide, resulting in steady, sustained release over time
of adults with epilepsy have at least 4 other chronic conditions2
of patients with epilepsy take at least 5 multiple medications, 3x higher than the general population (15%)4,5
Younger adults with a busier lifestyle, including work and childcare6
| * | Psychosocial concerns, depression, severe headache, anxiety, migraine, learning difficulties, intellectual and developmental disabilities, and autism spectrum disorders.3,7 |
| † | Other non-CNS comorbidities include heart disease, stroke, hypertension, prediabetes, cancer, dermatitis, arthritis, emphysema, asthma, and ulcer.7 |
| CNS=central nervous system. |
INDICATION
MOTPOLY XR is indicated for adults and pediatric patients weighing at least 50 kg for treatment of partial-onset seizures and adjunctive therapy in the treatment of primary generalized tonic‑clonic seizures.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
INDICATION
MOTPOLY XR is indicated for adults and pediatric patients weighing at least 50 kg for treatment of partial-onset seizures and adjunctive therapy in the treatment of primary generalized tonic‑clonic seizures.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
MOST COMMON ADVERSE REACTIONS
The most common adverse reactions in adults (≥10% and greater than placebo) are diplopia, headache, dizziness, nausea, and somnolence.
USE IN SPECIFIC POPULATIONS:
Please refer to the full Prescribing Information and Medication Guide for MOTPOLY XR for additional important information.
References:
1. Data on file, Aucta Pharmaceuticals; 2022.
2. Centers for Disease Control. National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP). Epilepsy. https://www.cdc.gov/epilepsy/data-research/facts-stats/index.html. Accessed August 29, 2024.
3. Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512-521.
4. Terman SW, Aubert CE, Maust DT, et al. Polypharmacy composition and patient- and provider-related variation in patients with epilepsy. Epilepsy Behav. 2022;126(4):108428.
5. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
6. Mendorf S, Prell T, Schönenberg A. Detecting reasons for nonadherence to medication in adults with epilepsy: a review of self-report measures and key predictors. J Clin Med. 2022;11(15):4308.
7. Centers for Disease Control. Comorbidity in adults with epilepsy—United States, 2010. MMWR. 2013;62(43);849-865.